Home > Our Research > FXR
Farnesoid X Receptor (FXR)
FXR, a nuclear receptor expressed in the liver and intestine, is a key regulator of bile acid, inflammatory, fibrotic and metabolic pathways.
Understanding FXR
- FXR is a nuclear receptor found in the liver, intestine, kidney, and adipose tissue
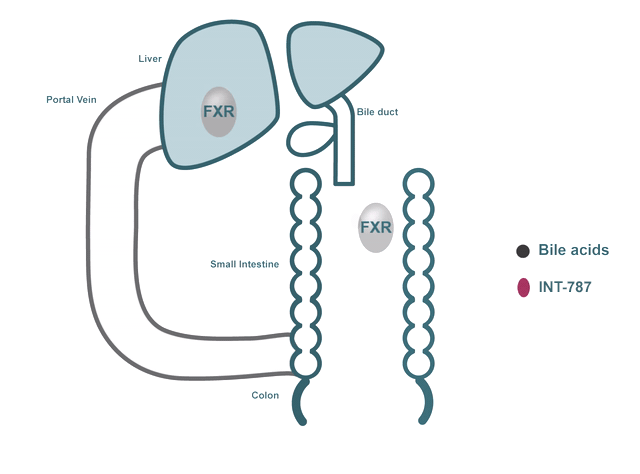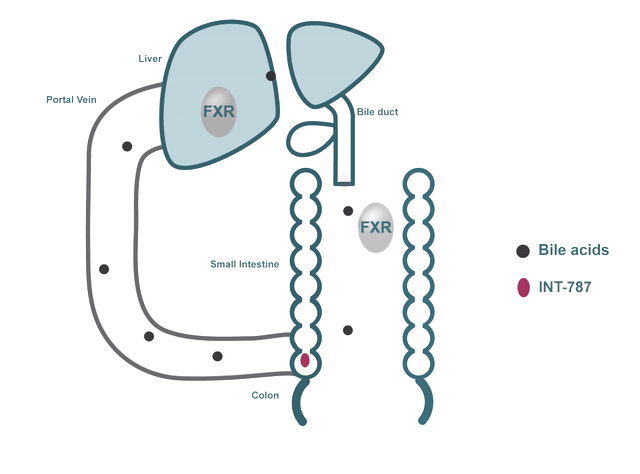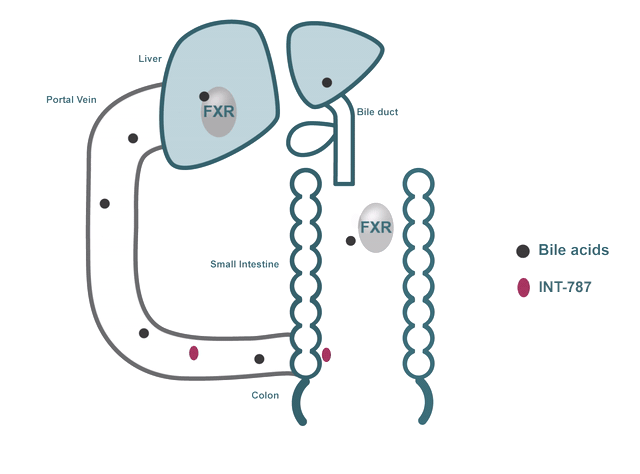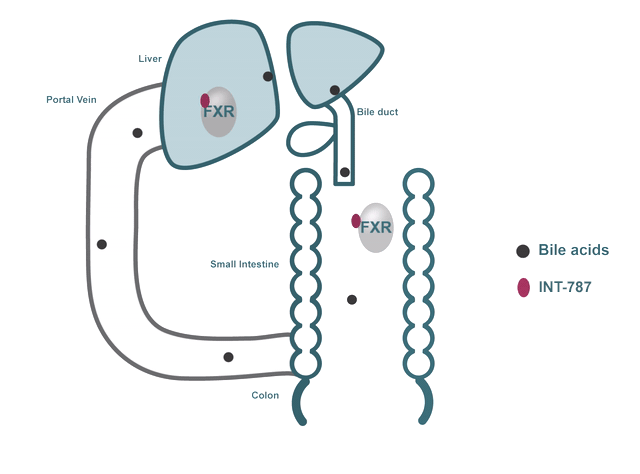
- FXR regulates many biological processes, including:
- Bile acid metabolism
- Inflammation
- Fibrosis
- Glucose metabolism
- Lipid metabolism
In addition to their role in digestion and absorption, bile acids have been shown to bind and activate dedicated receptors like FXR.
INT-787 is a clinical-stage next-generation FXR agonist that selectively targets the FXR pathway.
FXR Resources
- Recent insights into farnesoid X receptor in non-alcoholic fatty liver disease (Xu et al, World Journal of Gastroenterology, 2014)
- Pleiotropic roles of bile acids in metabolism (de Aguiar Vallim et al, Cell Metabolism, 2013)
- Physiological and molecular biochemical mechanisms of bile formation (Reshetnyak, World Journal of Gastroenterology, 2013)
- Bile acid transporters and regulatory nuclear receptors in the liver and beyond (Halilbasic et al, Journal of Hepatology, 2012)
- Deciphering the nuclear bile acid receptor FXR paradigm (Modica et al, Nuclear Receptor Signaling, Nov 2010)
- Role of nuclear receptors for bile acid metabolism, bile secretion, cholestasis, and gallstone disease (Claudel et al, Biochimica et Biophysica Acta, 2010)
- Role of bile acids and bile acid receptors in metabolic regulation (Lefebvre et al, Physiological Review, 2009)
- Bile acids as regulatory molecules (Hylemon et al, Journal of Lipid Research, 2009)
- The role of FXR in disorders of bile acid homeostasis (Eloranta et al, Physiology, 2008)
- Farnesoid X Receptor Agonists: A Promising Therapeutic Strategy for Gastrointestinal Diseases (Almeqdadi et al, Gastro Hep Advances, 2023)
- Identification of a nuclear receptor that is activated by farnesol metabolites (Forman et al, Cell, 1995)
- Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors (Seol et al, Molecular Endocrinology, 1995)
YOU ARE NOW leaving interceptpharma.com
You are now leaving Intercept Pharmaceuticals’ corporate website and entering a site intended for U.S. audiences only.
YOU ARE NOW leaving interceptpharma.com
You are now leaving Intercept Pharmaceuticals’ corporate website. Intercept does not control or endorse the content of this external site.
YOU ARE NOW leaving interceptpharma.com
You have selected a link that will take you to a site maintained by a third party who is solely responsible for its contents. Intercept provides this link as a service to its website visitors. Intercept is not responsible for the content or the privacy policy of any third party websites.
Close this window to return to Intercept Pharmaceuticals’ site or click ‘Continue’ to proceed.
United States (US)
European Union (EU)
Canada
Israel
Switzerland
Australia
Liechtenstein
United Arab Emirates (UAE)
Exploring Racial Differences and Disparities in PBC Care
Intercept Pharmaceuticals, Inc., a wholly owned biopharmaceutical subsidiary of Alfasigma S.p.A., has voluntarily withdrawn OCALIVA® (obeticholic acid) from the market for the treatment of primary biliary cholangitis (PBC), effective November 14.
Patients should talk to their healthcare professionals and may also contact Intercept's patient support services (Interconnect at 1-844-622-4278) through December 31, 2025.
Healthcare professionals can contact Medical Information at medinfo@interceptpharma.com or 1-844-782-4278. Additionally, patients or healthcare professionals may request Full Prescribing Information or the OCALIVA Medication Guide by contacting medinfo@interceptpharma.com.
For all other inquiries please contact: info@interceptpharma.com